Steel can be protected by cathodic protection if it has been submerged in the electrolyte (water, soil, concrete) and if the surface of the steel and volume of the electrolyte are significant. Often this is combined with coatings, such as for ships and buried pipelines. But for example offshore platforms and offshore windmills are successfully protected uncoated for >30 years without any sign of corrosion.
For electrochemistry, cathodic protection performance easily can be measured with the SensCorr by connecting the working electrode to the object under protection. This is done via the enclosure through a cable from this object to the enclosure. In this way the rest potential Ecp is measured between the working electrode and the reference electrode (both made of carbon steel). Normally E-cp is -300 – -500 mV St.. Also a silver/silver chloride or copper-copper sulfate reference electrode can be used. In that case E-cp is -800 mV – -1000 mV.
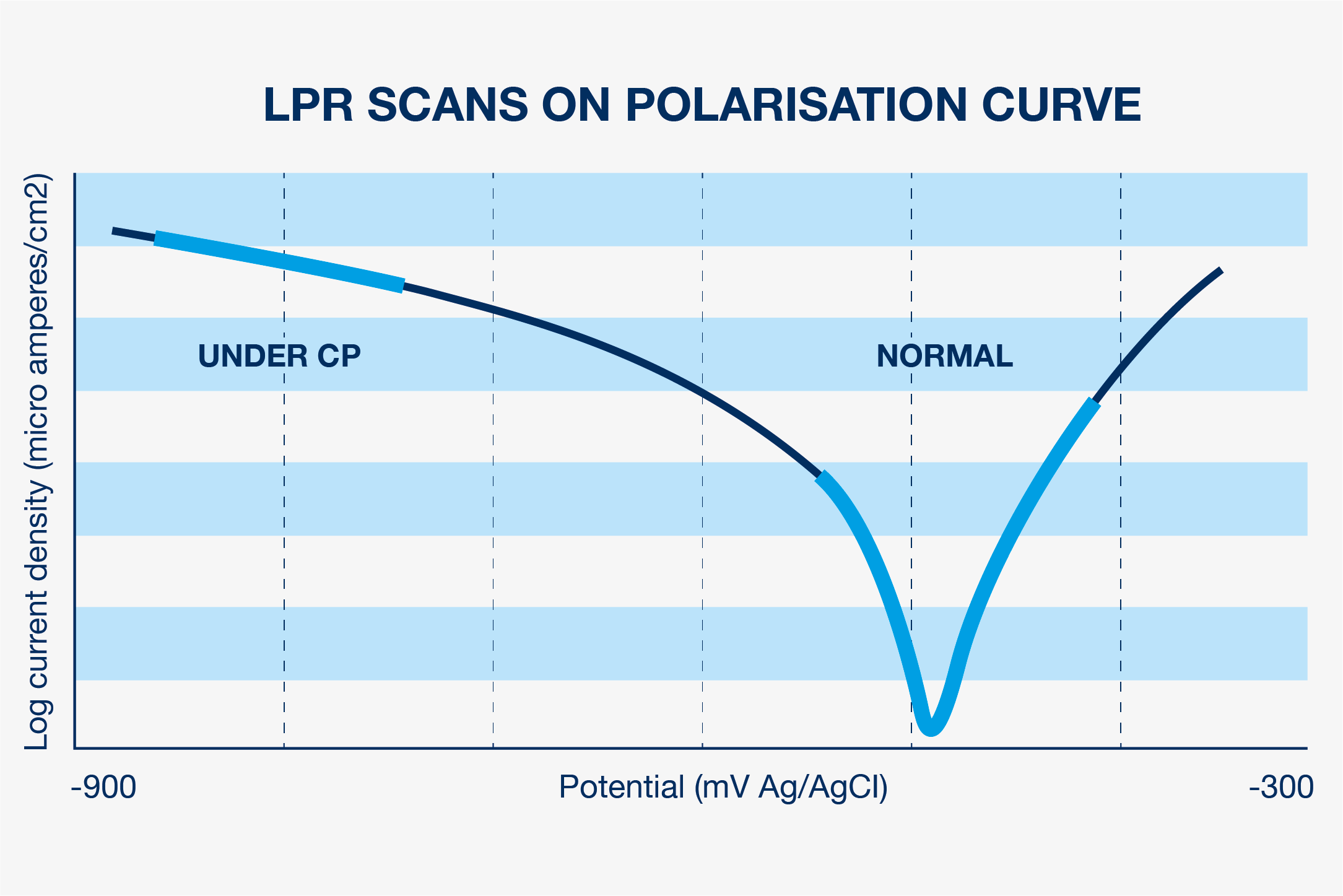
After, the object is disconnected from the working electrode and the current belonging to E-cp is measured. This is the CP current density in mA/cm2. See the graph below with a lpr scan of the working electrode ‘under cathodic protection’. This current, knowing the surface of the electrode, is the current density of the CP system. The current density is very much depending on the formation of a complex (protective) calcareous layer and biofouling on the steel surface.
For years the CP performance and AC interference of buried pipelines is measured with ER probes. The SensCorr measures ER with a sensitivity of 0,1 micrometer metal loss.
The interface (the HART Loop Converter) transfers 4 parameters:
- ER metal loss in micrometer.
- The potential E-cp in mV.
- The CP current density in mA/cm2.
- The potential E-corrosion in mV. This is the potential where the cathodic current reverses in an anodic current.


The SensCorr’s merit is the improvement of asset management. Currently the cathodic protection is checked by measuring the potential. The SensCorr is able to measure the potential without an IR Drop, because the reference electrode is placed right next to the working electrode (which is under cathodic protection). Even if the potential is correct, corrosion can arise due to AC interference or other stray currents. The added value of SensCorr is that you also can measure the current density, the corrosion potential and the presence of AC interference. In case the SensCorr detects corrosion through AC interference, the cathodic protection system can be adjusted (automatically) so that the corrosion is stopped.



